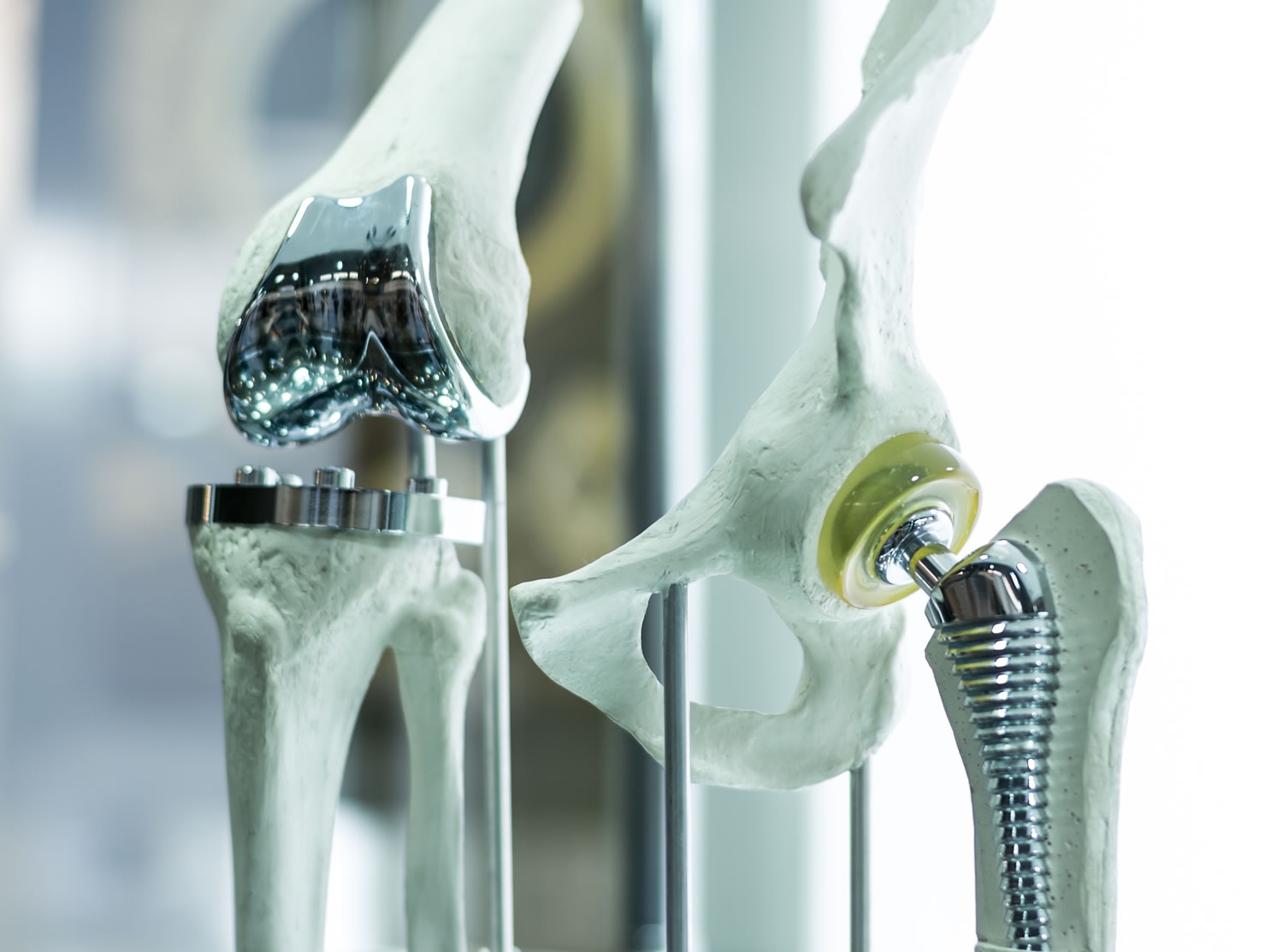
Quietly, over many decades, our aging bodies have undergone a revolution. Although we’ve not evolved, our science and technology have slowly transformed to assist our aging bodies. It was not so long ago that a broken hip consigned the elderly sufferer to a bath-chair for the remainder of their painful life. But now if you’re one of the fortunate few with advanced healthcare options, the hip can be fixed.
These same people can replace (as a fairly routine procedure) knees, eye lenses, or have implants to regulate heart rhythm or restore valve integrity. They likewise may soon have access to mechanical hearts, and potentially to interface thoughts directly with a computer.
But there’s one fact that this quiet revolution cannot escape. Contaminated, dirty implants will kill. However good that pacemaker is technically does not matter if it brings with it a contamination the body is ill-equipped to fight.
There are three kinds of contamination that need to be addressed, and they all represent hurdles that an implant supply chain must clear - often controlled and regulated through the entire supply chain to the raw material source.
- Viral, bacterial and fungal pathogens. Obviously anything that’s going into a patient's body mustn’t initiate an acute or chronic infection and add to the patient's burden of recovery.
- Toxic, particulate and reactive contaminants - manufacturing residues, environmental contaminants, packaging detritus etc - may pose an inflammatory or autoimmune trigger risk that could be as significant as a living pathogen.
- Leachant and spalling from materials in the implant, potentially pumping a steady stream into the patient.
In addition to these contaminants, any implant risks protein adhesion to alien materials, part of the typical Foreign Body Reaction (FBR) of inflammation and eventual fibrosis (enclosure by fibroblasts, when the body cannot destroy the foreign matter). As implantable technologies become increasingly refined devices, interfaced with ever more delicate and life critical tissues, the issues that develop around tissue-implant interaction by FBR become overwhelming [1]
And that's a lot to take on. So let’s examine the standards and how manufacturing and last-step preparation of various types of implants can be best handled.
Standards for Sterilization and Non-Pathogen Contamination
The earliest attempt to regularize the cleanliness and suitability of materials for surgical implants was a committee formed in 1972 by the ISO - the Technical Committee on Implants for Surgery (ISO/TC150) - which is still going strong. By the mid 1980s, 34 standards were published or in development, as reported in the Annals of the Royal College of Surgeons of England[2]. This article describes the development of clear standards, and recognises the need for a more systematic approach.
Correct clean fabrication and formulation, packaging, storage and sterilisation, suitable design, ease of application and removal, absence of toxic effects either from degradation of materials or from interaction with host tissues, the development of immunopathological or carcinogenetic effects—these are but some of the facts to be considered.
The dynamic forces to be encountered in function will influence the design of an implant and successful implantation. However, there is still empiricism here because little is known of the mechanical forces involved at living tissue level.
and…
The role of the manufacturer; The surgeon is indeed indebted to the manufacturer, who produces low-volume, high-cost items with all the fears and worries of biological application. Surgical progress would have been impossible without the many manufacturers of repute. Modern applications are more scientific than the earlier trial and error technique in solving problems of design and materials. The engineering sciences have become increasingly orientated to biological problems, and surgeons have welcomed this. The materials scientist, mechanical engineer, metallurgist, polymer scientist—these and others form the nucleus of an interdisciplinary approach to the problem. Their activities are just as welcome to the manufacturers. It was customary for the quality of a product to be accepted on the good name of a particular manufacturer. Indeed, this may still be the case, but in the last decades the number of manufacturing concerns in the 'medical' field has greatly increased. Many of these are no longer organisations with particular expertise but off-shoots of large combines covering a wide range of merchandise. This, in such a specialist field, can be a potentially hazardous situation. International trade/economics agreements have their effects. The medical products market has become commercially more attractive. Larger manufacturers have become multinational.
The ISO is progressively reducing empiricism by stringently applying the learning by trial-and-error outcomes that historically led surgical advances. More trials, less errors lead to better results and less disrupted development. And better patient outcomes.
The most important of the current standards is ISO 19227:2018(en) Implants for surgery — Cleanliness of orthopedic implants — General requirements. This standard specifies the stages of contaminant removal from the raw material to the packaged product stage for orthopedic implants which is likely the largest sector.

Made with a variety of components and materials, from precise PTFE moldings to drop-forged and CNC-machined titanium and advanced alloy stainless steel parts, with parts undergoing chemical and mechanical polishing and surface treatments/texturing to promote bone adhesion, the implant manufacturing process has many points which may introduce contaminants.
Foreign materials may include metal oxide flakes, cutting coolants, machine oil, depolymerized and scorched plastic microparticles. Then there’s human handling for setup and inspection, and there’s the contaminated-at-source risks with polymers, alloys, packaging materials and more.
Standards relating to general surgical equipment and implant cleaning and sterilization are extensive, and focus heavily on the processes of validation that can be used to demonstrate conformance to the standards [3].
- The ICS (International Classification of Standards) 11.040.40 Implants for Surgery, prosthetics and Orthotics (including pacemakers).
includes ISO 19227:2018 Implants for surgery — Cleanliness of orthopedic implants — General requirements. - ISO 14937:2009(en) Sterilization of health care products — General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices.
- ISO 10933 - Biological Evaluation of Medical Devices outlines evaluation methods for biocompatibility and assessment of leachant or contaminant issues.
- GMP 21 CFR 820 is the FDA standard that covers Quality System Regulation for medical device manufacture, which includes implanted devices.
These standards, and many more regional standards derived from them, control the state of preparedness of ‘passive’ mechanical implants like prosthetic joints, stents, meshes, heart valves plus ‘simpler’ (i.e. less intrusively interfaced) electronics.
Specialist standards for manufacture of ocular implants have distinct rule-books which also have FDA and EU GMP (Good Manufacturing Practice) systems that support them:
- ISO 11979-10:2018 Ophthalmic implants — Intraocular lenses — Part 10: Clinical investigations of intraocular lenses for correction of ametropia in phakic eyes.
- ISO 11979-2:2014(en) Ophthalmic implants — Intraocular lenses — Part 2: Optical properties and test methods.
The preparation of these implants for use is an active area of research, including specialist standards for active (electronic) implantable devices. These cover the full range of currently envisaged devices - including neurological input/output devices and artificial hearts that are currently experimental.
- Guidelines for the cleaning and sterilization of intraocular surgical instruments [4].
- ISO 14708-1:2014 Implants for surgery — Active implantable medical devices — Part 1: General requirements for safety, marking and for information to be provided by the manufacturer.
- ISO 14708-2:2019 Cardiac pacemakers; ISO 14708-3:2017 Implantable neurostimulators; ISO 14708-4:2008 Implantable infusion pumps; ISO 14708-5:2020 Circulatory support devices; ISO 14708-6:2019 devices intended to treat tachyarrhythmia (including implantable defibrillators); ISO 14708-7:2019 cochlear and auditory brainstem implant systems.
Intermediate Cleaning (During Manufacture)
During manufacture, the intermediate cleaning processes are not specified by the standards - only their outcomes, in terms of inorganic and organic residues. The most watched-for potential contaminants are particulates and oils.
There are simply too many materials in the range of implants, too many potential contaminants, too many industrial processes that serve as post process cleaning and preparation for the next stage, so the standards only specify outcomes. However, as the implant sector blossoms into its next stage - of increased delicacy and integration into the patient, of increased intricacy of operation and therefore greater potential for insidious harm - the standards will doubtless continue to evolve along with the market.
Forged titanium alloy and stainless steel parts - these will likely get their first cleaning before being forged from a pre-cast blank or billet - the post furnace oxide build-up will be removed with a wire brush or a water blast, to reduce tool wear and to prevent embedding of oxide flakes and detritus into the forged surface. This will be repeated between forging stages, if the part is re-heated, re-establishing the oxide film.
Once forging is complete, some of the surfaces of the implant may have their final texture/shape and require no further processes. However, most will require machining, plating, polishing or a number of other potential processes, with further intermediate cleaning processes. This will likely involve roller cleaning, sandblasting cleaning, shot blasting cleaning or acid pickling.
CNC machining processes - all metal cutting processes require coolants - sometimes oil emulsions in water, sometimes complex hydrocarbons, but all represent contaminants that must be removed entirely.
Post machining, this decontaminant phase can be performed by;
- Ultrasonic bath cleaning, with a low concentration degreasing agent. The cavitation effect of the ultrasonic agitation pulls fluid off the contaminated surfaces in explosive bubbles, amplifying the effect of surfactant agents added to the bath.
- Steam cleaning, with surfactant agents.
- Vacuum bath cleaning, pulsating vacuum to create cavitation at the material surface
- Solvent steaming, suspending parts in a hot vapor atmosphere which condenses on them and then drips off, carrying contaminants away.
Polishing processes - bearing surfaces of joint implants must be brought to a high polish in order to maximize joint life. For example, a hip socket is generally considered to have a maximum 10-year operational life - which would be considerably shortened by a surface irregularity on the ball component.
The most common method used is electropolishing, which uses an electrolytic solution to erode high-points selectively as current flows in the solution. The electrolyte is by nature water soluble, so cleaning requires ultrasonic or vacuum washing with clean (generally deionized or distilled) water and mild surfactants, followed by rinsing.
Molded parts - there are strict operational guidelines in the standards for selection of and utilization of materials for implants - more so for polymers than for metals, because of the greater potential for interstitial impurities that become time-delayed contamination as they leach out, often accelerated by the biological processes active around the implant.
Use of antioxidants, plasticizers, and mold-release agents are banned and stringent restrictions are applied to the molding conditions, to ensure a minimum of entrained polymer detritus and partially or fully depolymerised residues from scorching. While PTFE compounds are more chemically resilient than, for example, UHMWPE (Ultra High Molecular Weight PolyEthylene), these issues can and do arise with any polymer, consequent upon efforts to reduce manufacturing costs.
Silicone rubber components - such as intra ocular devices for cataract surgery - are generally manufactured from platinum-catalyzed monomers and can withstand all but the most aggressive cleaning and sterilization procedures, so despite their general softness they can be put through similar post-moulding processes as harder, more durable plastic components.
Some factors cannot be cleaned once integrated into the molded part, so they must not be present - but the potential for traces of machine oils and loose polymer particulates can be addressed with ultrasonic cleaning using a mild phosphoric acid solution, combined with a surfactant. This is standard surface preparation for medical grade plastics (and for electroplating or non-solvent based painting systems for non-medical grade parts as well).
Electronic implants - Implanted electronics are covered in the existing standards, to the degree that they’re currently established and used - cochlear implants, neural activity monitors, pacemakers and diffusion pumps. These devices are assumed to be entirely enclosed and largely shut away from tissue contact. They’re treated as manufactured polymer/metal/ceramic components that entirely enclose any electronic elements, other than wire traces that run from the device to tissues that will be subjected to electrical stimulation, or from which signals will be collected, or both [5].
On that basis, they are well covered in GMP approaches which the ISO and client bodies define very clearly.

Final Sterilization
Despite multiple cleaning processes applied to components of implants as they’re manufactured, no product is going to be surgically introduced into a patient without that final stage of sterilization.
This is required to be performed by an established method that is extensively validated to destroy pathogens on surgical instruments and equipment. The standards apply just as well to devices that are intended to be left inside the patient.
These processes include;
- Gamma and UV irradiation - brutal, not without hazards when equipment malfunctions, but highly effective. Quite harmful to delicate electronics.
- Autoclave pressure/steam - a less worrying process and the original methodology, with a long track record. Mostly non damaging, but some necessary materials may be challenged by the temperatures required.
- Ethylene oxide exposure - a highly effective technique, using a toxic gas that is anathema to all life. This is a very effective technique and the gas is effectively harmless to inert materials, making it very well suited to more delicate devices.

As a rule, the manufacturing process for surgical implants ends with one of these processes and then sealing into sterile and dependable packaging. In reality, the more delicate the device, the more likely it is that it is manufactured under clean room and positive pressure filtered air conditions preventing contamination in the first place.
Products that arrive as sterile, from a certified and reliable source, will be used as such and trusted. Breakdowns in these processes do happen, but not often - suggesting the application of the ISO standards and the local GMP guidelines are followed methodically.
Is It Enough?
Like all standards, these transfer the knowledge of people doing the job into best practice guides and rules. That means they are never at the leading edge, always aspiring to adequacy, and reaching towards versions of the future that won’t eventuate in the way we imagine today. But they do a workable and largely effective job in most cases. Some research suggests gaps and overlaps that can be addressed [6];
CONCLUSIONS: ISO 19227 is very helpful in the development and validation of a cleaning process. The proposed analytical methods TOC and THC can be potentially useful for process control of an established cleaning process.
However, the list of possible test methods is not sufficiently comprehensive. Focusing on
specific examples of test methods and giving suggestions on how to derive an acceptance criterion could give a false sense of security. A general acceptance limit of 0.5 mg/implant [note: of contaminant] is not appropriate, as the size, complexity, manufacturing and cleaning process of implants are diverse. Thus, limit values have to be determined in a toxicological risk assessment and are usually supplemented by the requirement that the device is visually clean. Therefore, we suggest that the chemical
characterisation of orthopaedic implants should be performed according to ISO 10993-18.
Surface contamination is then considered and evaluated as part of the chemical
characterisation. If necessary according to the risk mitigation plan, even small amounts of contamination should be identified and quantified. If the toxicological assessment shows that the surface contamination is problematic, the cleaning process must be improved in accordance to ISO 19227.
What’s Next
What is paramount is that these implants are cleaned fully, at all stages of the manufacture, preparation, and surgical processes. Of the various approaches available, vapor cleaning is a gold standard that is well-validated and ideally suited for most applications. Of the manufacturers for these azeotropic and near-azeotropic solutions, Chemtronics has been an industry leader for decades serving many industries including medical device manufacturing. These solvents boil and the vapor rises to clean the surface(s) completely, leaving no contaminants or soil in their wake. Ideal selections are from our Tri-V High Performance Solvent line, including Electro-Wash Tri-V, Max-Kleen Tri-V, and Flux-Off Tri-V. Contact us for identifying the perfect choice for your application.
References
[1] Salatino et al. (2017). Glial Responses to Implanted Electrodes in the Brain. Abstract. Retrieved from https://pubmed.ncbi.nlm.nih.gov/30505625/
[2] Bloch, B. (1984). International standards for surgical implants. Ann R Coll Surg Engl, 66(5): 369-71. PMID: 6486676; PMCID: PMC2493666.
[3] ISO (varied). 19227:ed-1:v1:en. Implants for surgery — Cleanliness of orthopedic implants — General requirements. Retrieved from https://www.iso.org/obp/ui/#iso:std:iso:19227:ed-1:v1:en August 2022.
[4] Chang et al (2018). Guidelines for the cleaning and sterilization of intraocular surgical instruments. Journal of Cataract & Refractive Surgery, 44(6): 765-773.
[5] Spearman et al., (2018). Tissue-Engineered Peripheral Nerve Interfaces. Abstract. Retrieved from https://onlinelibrary.wiley.com/doi/10.1002/adfm.201701713
[6] Rohrer et al (2021). Cleanliness of Orthopaedic Implants According to ISO 19227: Differences and Gaps Compared to ISO 10993-18. Abstract. Retrieved from https://www.rms-foundation.ch/fileadmin/dokumente/MtE/Poster_2021/Abstract_P13_Rohrer-Wirz.pdf
Ask A Technical Question
Stay up-to-date on Chemtronics news, products, videos & more.